Moderna can meet 2023 COVID forecast, analysts suggest, while Pfizer won't.
Moderna expects to hit lower end of sales target for 2020, only needing to tap a small portion of potential markets.
November 1st 2023.
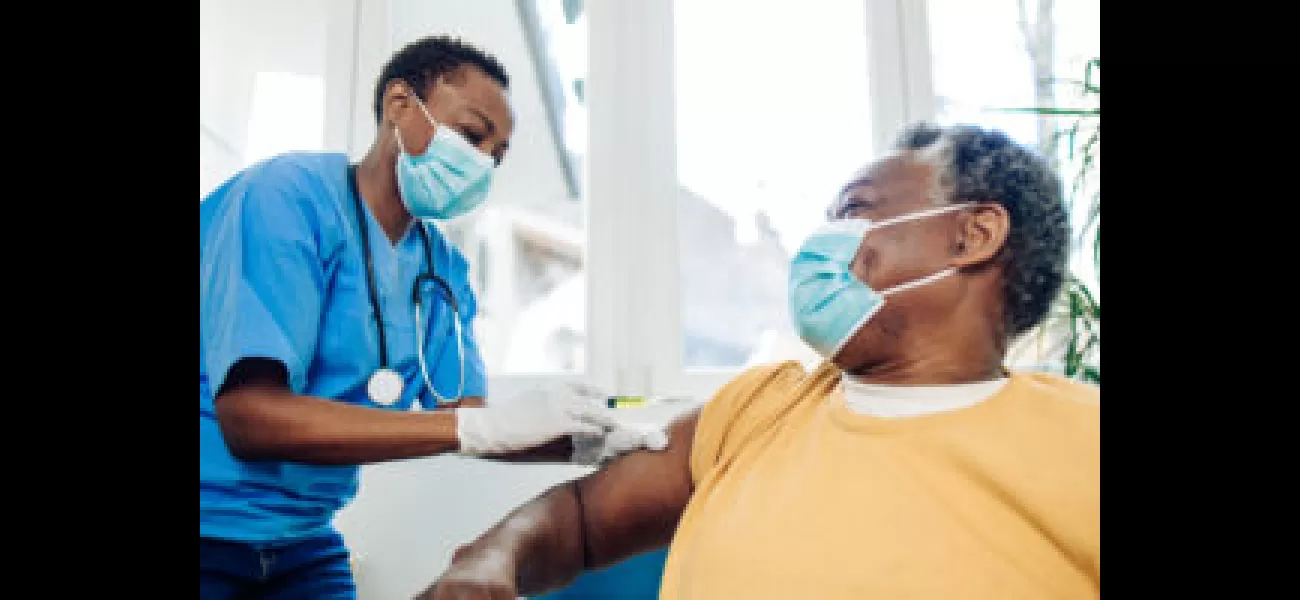
Moderna has announced they expect to hit the lower end of their sales target for 2021. Industry analysts estimate that around 20 million people need to be vaccinated with Moderna's updated COVID-19 vaccine for the company to reach $2 billion in 2023 sales from the private market, which is a figure many analysts believe is attainable. Moderna has previously stated they anticipate the demand for their COVID vaccine to reach up to 100 million doses in the fall season.
Yet, their goal was called into question last month when Pfizer lowered their full-year outlook for sales of their COVID-19 shot by around $2 billion due to lower-than-expected vaccination rates. This has caused Moderna's shares to drop by 22%. Analysts have said that it is unlikely Moderna will have a negative fall like Pfizer, because they started off much more conservatively.
Jefferies analyst Michael Yee has also noted that while the rollout of the new shots has been slow, it seems to be picking up. He believes that most of the demand will come from people aged 65 and over. Moderna will be releasing their third-quarter results on Thursday, and two days prior to that, Pfizer posted their first quarterly loss since 2019. This was due to a large charge to account for the U.S. government returning millions of doses of their COVID-19 antiviral treatment, as well as inventory of their COVID vaccine.
Moderna's COVID vaccine is currently the only product they have on the market. Research and development costs have ballooned 62% to $1.1 billion in the second quarter as they seek to bring other products to market, including a flu vaccine and a shot against respiratory syncytial virus. Moderna's RSV vaccine is expected to launch in the United States in 2024 and has been found to be 82.4% effective in older adults with three or more symptoms in a late-stage trial. It will be competing with recently approved vaccines from Pfizer and GSK.
Data from a late-stage study of Moderna's flu vaccine with an updated formulation released in September showed it generated a stronger immune response against all four A and B strains of the influenza virus compared to traditional flu shots. Moderna's broader mRNA based respiratory pipeline, which includes RSV and flu vaccines, is expected to reach $10 billion to $12 billion in sales by 2025, which will reduce expenses and bring R&D stability.
Yet, their goal was called into question last month when Pfizer lowered their full-year outlook for sales of their COVID-19 shot by around $2 billion due to lower-than-expected vaccination rates. This has caused Moderna's shares to drop by 22%. Analysts have said that it is unlikely Moderna will have a negative fall like Pfizer, because they started off much more conservatively.
Jefferies analyst Michael Yee has also noted that while the rollout of the new shots has been slow, it seems to be picking up. He believes that most of the demand will come from people aged 65 and over. Moderna will be releasing their third-quarter results on Thursday, and two days prior to that, Pfizer posted their first quarterly loss since 2019. This was due to a large charge to account for the U.S. government returning millions of doses of their COVID-19 antiviral treatment, as well as inventory of their COVID vaccine.
Moderna's COVID vaccine is currently the only product they have on the market. Research and development costs have ballooned 62% to $1.1 billion in the second quarter as they seek to bring other products to market, including a flu vaccine and a shot against respiratory syncytial virus. Moderna's RSV vaccine is expected to launch in the United States in 2024 and has been found to be 82.4% effective in older adults with three or more symptoms in a late-stage trial. It will be competing with recently approved vaccines from Pfizer and GSK.
Data from a late-stage study of Moderna's flu vaccine with an updated formulation released in September showed it generated a stronger immune response against all four A and B strains of the influenza virus compared to traditional flu shots. Moderna's broader mRNA based respiratory pipeline, which includes RSV and flu vaccines, is expected to reach $10 billion to $12 billion in sales by 2025, which will reduce expenses and bring R&D stability.
[This article has been trending online recently and has been generated with AI. Your feed is customized.]
[Generative AI is experimental.]
0
0
Submit Comment